Overcoming the diagnostic and therapeutic challenges of canine immune-mediated thrombocytopenia
Immune-mediated thrombocytopenia is the most common acquired cause of abnormal primary hemostasis in dogs.
Platelets play an essential role in normal hemostasis. In primary hemostasis, platelets bind to the damaged vessel wall, forming an initial platelet plug. The platelet plug is then stabilized by fibrin, which is generated through the activation of clotting factors in secondary hemostasis.
Immune-mediated thrombocytopenia is the most common acquired cause of abnormal primary hemostasis in dogs.1 It involves antibodies binding to the surface of platelets, causing premature platelet destruction by macrophages.2 Besides increased platelet destruction, other non-immune-mediated mechanisms of thrombocytopenia include decreased platelet production by the bone marrow, platelet sequestration (primarily in the spleen), and increased platelet consumption or loss.3
Immune-mediated thrombocytopenia is often diagnostically and therapeutically challenging. Understanding the underlying pathophysiology and principal differential diagnoses for thrombocytopenia in dogs is vital to develop an appropriate diagnostic and therapeutic approach.
PATHOPHYSIOLOGY
Platelets are produced from the fragmentation of bone marrow megakaryocytes and are then released into the circulation where their average life span is eight to 12 days. Aged platelets are removed from the circulation by tissue macrophages, especially in the spleen.1,4 Immune-mediated thrombocytopenia is mainly a disorder of accelerated platelet destruction.2 In this disease, antibodies bind to platelet membranes, enhancing platelet clearance by the mononuclear phagocytic system. This process usually occurs in the spleen although the liver also plays a role.1,2 Thrombocytopenia develops as platelet destruction exceeds compensatory production by the bone marrow.1
Antiplatelet antibodies are directed against normal platelet surface antigens.2 Platelet integrin alphaIIBbeta3 (formerly glycoprotein IIb/IIIa) on the platelet surface is highly immunogenic and is thought to be the most frequent target antigen.5 Platelet-surface-bound antibodies in dogs with immune-mediated thrombocytopenia are frequently immunoglobulin G (IgG).2 Antiplatelet antibodies may also alter platelet function and further compromise primary hemostatic function.1,5 However, the clinical relevance of this altered platelet function is unclear since immature platelets have increased hemostatic function that may compensate for the platelet dysfunction.1
Immune-mediated thrombocytopenia can be classified as primary or secondary.1
Primary immune-mediated thrombocytopenia
Primary immune-mediated thrombocytopenia has also been called idiopathic thrombocytopenic purpura. In this spontaneous autoimmune disorder, autoantibodies are directed against specific portions of the platelet membrane. It occurs in the absence of an underlying disease1,2 and can only be diagnosed once causes of secondary immune-mediated thrombocytopenia have been ruled out.6
Secondary immune-mediated thrombocytopenia
In secondary immune-mediated thrombocytopenia, antibodies bind to antigens adsorbed to the platelet surface or immune complexes nonspecifically bind to the platelet.5 Secondary immune-mediated thrombocytopenia occurs as a result of an underlying disorder.1,2 Many underlying causes of secondary immune-mediated thrombocytopenia have been reported. Often there is an association between a disease and thrombocytopenia, but the causative role has not been confirmed experimentally.1 Conditions that have been associated with immune-mediated thrombocytopenia include autoimmune disorders, drug therapy, blood product transfusion, vaccine administration, various neoplasms, and infectious agents.
Autoimmune diseases. Immune-mediated thrombocytopenia may occur in association with other autoimmune diseases in dogs such as systemic lupus erythematosus, rheumatoid arthritis, and immune-mediated hemolytic anemia.3
Drug and blood product therapy. Although any drug could provoke this disease, several drugs have been associated with immune-mediated thrombocytopenia in dogs, including auranofin (gold salts), cefazedone, and trimethoprim-sulfonamide combinations.1,2 Drugs generally act as haptens, which combine with platelets to form a drug-platelet complex that is antigenic.7 Drug-related immune-mediated thrombocytopenia usually develops weeks to months after initial therapy, resolves within two weeks of discontinuing therapy, and does not recur unless the drug is readministered.2,8 Severe thrombocytopenia caused by the production of antiplatelet antibodies has been reported in dogs within weeks after blood product transfusion.3
Vaccine administration. Canine postvaccinal immune-mediated thrombocytopenia has been suspected, but evidence of a direct causative role of vaccines in immune-mediated thrombocytopenia is lacking.1
Neoplasia. Thrombocytopenia is commonly associated with various hematopoietic and solid neoplasms, including lymphoma, mammary adenocarcinoma, mast cell tumor, hemangiosarcoma, nasal adenocarcinoma, and fibrosarcoma.1,9 Antibody against tumor antigens that are closely related to platelet membranes may initiate platelet destruction. In some dogs with solid neoplasms, thrombocytopenia resolves after tumor remission.1
Infectious agents. Viral, bacterial, rickettsial, protozoal, and parasitic infections may also play a role in inducing immune-mediated thrombocytopenia.2 Infections may result in immune-mediated platelet destruction by exposing antigenic sites on platelet surfaces or through immune-complex injury to platelet membranes.7 Antibodies that can bind to platelets have been identified in dogs with ehrlichiosis, babesiosis, leishmaniasis, and dirofilariasis.2,7,10 The pathogenesis of thrombocytopenia with several infectious diseases is multifactorial, involving decreased production by the bone marrow and splenic sequestration, in addition to immune-mediated destruction.8
NON-IMMUNE-MEDIATED CAUSES
When evaluating a patient with suspected immune-mediated thrombocytopenia, be sure to exclude non-immune-mediated causes. Other mechanisms of thrombocytopenia include decreased platelet production due to bone marrow disorders, platelet sequestration, non-immune-mediated platelet destruction, platelet consumption, and platelet loss. The cause of thrombocytopenia may be multifactorial.
Neoplasia may cause thrombocytopenia through immune-mediated and non-immune-mediated mechanisms. Non-immune-mediated mechanisms include platelet consumption, splenic sequestration, hemorrhage, myelophthisis, and bone marrow suppression by chemotherapy or radiation.2,8
Hemolytic uremic syndrome, a rare cause of thrombocytopenia, is readily differentiated based on renal failure, microangiopathic hemolytic anemia, and fever.2 Decreases in platelet number resulting from splenic sequestration are generally modest.2 Similarly, thrombocytopenia resulting from blood loss is generally mild and transient, although moderate to severe thrombocytopenia has been reported with anticoagulant rodenticide intoxication.8,11 Immune-mediated thrombocytopenia usually causes severe thrombocytopenia—often < 50,000 platelets/μl.1
A severe, inherited thrombocytopenia has been reported to occur in up to 50% of Cavalier King Charles spaniels.12 Platelet counts can be as low as 20,000/μl, but many affected dogs have macrothrombocytes, and this disorder is not associated with a bleeding tendency. Perform manual platelet counts in dogs of this breed with thrombocytopenia, as large platelets may result in falsely low platelet counts as measured by impedance analyzers.
Bone marrow disorders can cause thrombocytopenia, but if thrombocytopenia is the sole abnormality on a complete blood count (CBC), bone marrow disorders are unlikely. With the exception of early estrogen toxicity, bone marrow disorders that cause thrombocytopenia typically cause concurrent leukopenia, with or without anemia.1,2 The presence of a marked, nonregenerative anemia or neutropenia could suggest bone marrow disease; alternatively these findings may be seen with immune-mediated disease.
DIAGNOSTIC APPROACH
Signalment
Middle-aged dogs are most commonly affected by immune-mediated thrombocytopenia, but the disease can occur in dogs of any age. A high prevalence has been reported in miniature, toy, and standard poodles; cocker spaniels; Old English sheepdogs; and German shepherds, but any breed, including crossbreeds, may be affected.1,2,6 The high prevalence in some breeds may suggest a genetic predisposition. Although dogs of either sex may develop immune-mediated thrombocytopenia, about twice as many female dogs are affected compared with male dogs.1,2
Clinical presentation
Dogs with immune-mediated thrombocytopenia are often not presented for evaluation of bleeding, but rather for nonspecific clinical signs such as lethargy, weakness, or anorexia.1,2,6 Some affected dogs are asymptomatic. In these dogs, a low platelet count on a CBC may be the first indication of immune-mediated thrombocytopenia. Dogs with secondary immune-mediated thrombocytopenia could present for evaluation of clinical signs related to the underlying disease (e.g. peripheral lymphadenopathy with lymphoma or dyspnea with disseminated histoplasmosis).6 Other dogs with immune-mediated thrombocytopenia have signs of hemorrhage with a history of otherwise being apparently healthy.1 Unexpected hemorrhaging after trauma or routine surgery, is also a manifestation of immune-mediated thrombocytopenia.1,7

Table 1. Clinical Signs Typical of Primary and Secondary Hemostatic Disorders
Bleeding related to immune-mediated thrombocytopenia is typical of that expected with primary hemostatic disorders (Table 1). Bleeding from mucous membranes may cause gingival, nasal, conjunctival, preputial, penile, or vaginal hemorrhage.1,6 Petechiae or ecchymotic hemorrhages on the mucous membranes and skin, especially on the ventral abdomen, are typical (Figure 1).1,2,6,7 Mucous membranes may be pale if concurrent anemia is present. Gastrointestinal (GI) hemorrhage can manifest as hematemesis, melena, or hematochezia.1,6,7 Hyphema and retinal hemorrhages may be present, possibly causing blindness.1,2,7 Hematuria may result from hemorrhage into the urinary tract. Often, dogs with immune-mediated thrombocytopenia are otherwise clinically stable.1
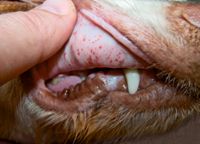
1. Petechial hemorrhages on the mucous membranes of a dog with immune-mediated thrombocytopenia.
Life-threatening hemorrhage can develop when bleeding occurs into the central nervous system, GI tract, or pulmonary parenchyma.2,13-15 Because of the potential for bleeding in any dog with immune-mediated thrombocytopenia, consider each case a serious and potentially life-threatening disorder, regardless of the signs on presentation. Severe GI hemorrhage is the predominant cause of death in dogs with acute immune-mediated thrombocytopenia.1
History and physical examination
Evaluation of patients with immune-mediated thrombocytopenia begins with a thorough history to identify suspected underlying causes such as medications or recent vaccinations (e.g. given within the previous month).8 Identify and discontinue the administration of medications that decrease platelet function (e.g. aspirin). Ask about travel to areas endemic for diseases such as ehrlichiosis and dirofilariasis, and, in endemic areas, inquire about the use of flea, tick, and heartworm preventives and the prior presence of ticks on the animal.8 Signs of other concurrent illness may be identified in the history or during the physical examination. Determine the location, duration, and severity of bleeding episodes. Also obtain a history regarding previous blood transfusions.
Perform a complete physical examination, including an ophthalmic examination. Note any evidence of abnormal hemostasis. A rectal examination may be required to obtain feces to evaluate for melena. Mild fever is present in some dogs with immune-mediated thrombocytopenia.1,13 Splenomegaly may be present,2,13 and some authors suggest that finding splenic enlargement on physical examination increases the likelihood of a concurrent disorder.1,2
Initial sample collection
At presentation, stabilize the patient, if necessary, and then initiate diagnostic testing. But before initiating therapy, collect and save EDTA, sodium citrated, and serum samples.6 Use these samples to perform a CBC with blood smear evaluation, a serum chemistry profile, a coagulation profile, heartworm antigen testing, and a crossmatch, if appropriate, as well as to measure antibody titers for infectious diseases. Collection by atraumatic venipuncture is necessary to avoid activating tissue factor within the needle. Process the samples within a few hours of collection to minimize platelet clumping. If severe thrombocytopenia is suspected, it is vital that the patient be handled as carefully as possible to avoid iatrogenic hemorrhage.
Evaluating the CBC and blood smear
Confirm thrombocytopenia with a CBC that includes an automatic platelet count and a blood smear examination.6 Reference ranges for platelet counts vary slightly among laboratories but are typically between 200,000 and 500,000/μl.1 Healthy greyhounds and possibly Shiba Inus have lower platelet numbers than other breeds.16 Spuriously low platelet counts can be due to clumping. A low count can also occur when macroplatelets are present and overlap with the size of erythrocytes. Most bench-top hematology analyzers use cell size, measured by impedance technology, to distinguish platelets from erythrocytes. So when they overlap, the analyzer has difficulty distinguishing the cell type, and platelets can be counted as erythrocytes. Consequently, low platelet counts should always be verified by examining a blood smear.2,6
A quick way to evaluate the number of platelets on a blood smear is to count the number of platelets per high-power field (HPF) (100X). Average the number of platelets in five to 10 fields, and then multiply the average number of platelets per HPF by 15,000/μl to calculate the total platelet count.6-8 More than 12 platelets per HPF represents a normal count.7,8 In addition, examine the feathered edge of the smear for platelet clumping. If platelet clumps are present, the platelet number assessment will be inaccurate.6,8 If clumping cannot be prevented, we recommend submitting the slide to a clinical pathologist for review and estimation of whether platelet numbers are adequate.
Also assess platelet size, as large, immature platelets (macrothrombocytes) indicate bone marrow response and have increased hemostatic function.1,6 On the other hand, small platelets (microthrombocytes) are suggestive of immune-mediated platelet destruction.2,5 However, platelets swell in EDTA, which may obscure this finding.5 Mean platelet volume (MPV) reflects the average size of platelets in circulation.4
The likelihood of hemorrhage increases when the platelet count is below 50,000/μl. However, spontaneous hemorrhage is uncommon until the platelet count is below 30,000/μl, unless a concurrent disorder of primary hemostasis, such as a platelet function defect or vasculitis, is present.1,6 Reticulated platelets, which are platelets recently released from the bone marrow, may be quantitated by using flow cytometry as an indication of thrombopoiesis, but this test is not routinely evaluated in general practice.4
Anemia may be present because of hemorrhage or concurrent immune-mediated hemolytic anemia (Evans' syndrome). About 20% of dogs with immune-mediated thrombocytopenia have concurrent immune-mediated hemolytic anemia.2 The presence of spherocytes or agglutination on a blood smear is helpful in diagnosing concurrent immune-mediated hemolytic anemia in an anemic patient with thrombocytopenia. Schistocytes may be present in patients with Evans' syndrome and concurrent disseminated intravascular coagulation, but their presence should also raise the index of suspicion for splenic hemangiosarcoma and can be seen with other conditions such as vasculitis.17
Leukocyte counts may be normal, or a stress leukogram may be present. In some patients with concurrent immune-mediated hemolytic anemia, a marked neutrophilia with a left shift may be present.2 Patients with immune-mediated thrombocytopenia generally have a less pronounced inflammatory leukogram; a marked neutrophilia, left shift, or toxic change should be evaluated for underlying inflammatory disease.
Additional diagnostic testing
Achieving a definitive diagnosis of immune-mediated thrombocytopenia can be challenging, because no single test is diagnostic.6 The diagnosis is primarily one of exclusion, thus, other causes of thrombocytopenia should be ruled out (Table 2).2,8

Table 2. Causes of Canine Thrombocytopenia
Blood testing and urinalysis. In addition to a CBC, perform a serum chemistry profile and urinalysis (collected by free catch) as part of a minimum database.8,13 Consider heartworm testing.8 Perform serum antibody titers to rule out rickettsial and fungal disorders based on the geographical location and travel history. Anaplasma platys (formerly Ehrlichia platys) causes a severe cyclic thrombocytopenia in dogs, but the dogs are usually asymptomatic.6 Measure prothrombin time, an activated partial thromboplastin time, and fibrin degradation products or D dimer concentrations to determine whether the thrombocytopenia is accompanied by other hemostatic abnormalities.6
Clinical pathology abnormalities that suggest a consumptive coagulopathy (disseminated intravascular coagulation) include schistocytes, prolonged coagulation times, elevated fibrin degradation products or D-dimer concentrations, and decreased fibrinogen concentrations, particularly in patients with concurrent clinical illness.2,8 Activated clotting time can be falsely prolonged if the platelet count is < 10,000/μl.1,6 A buccal mucosal bleeding time assesses primary hemostasis and will be prolonged in patients with very low platelet counts (e.g. < 30,000/μl). Thus, this test is not indicated in patients with marked thrombocytopenia.
Imaging. Evaluate for underlying neoplasia by performing thoracic and abdominal radiography and abdominal ultrasonography.8 Obtain fine-needle aspirates of enlarged organs, such as the liver, lymph nodes, and spleen, for cytologic examination if the platelet count is adequate (> 50,000/μl); otherwise, such sampling is unsafe.6
Bone marrow aspiration. If concurrent leukopenia (with or without anemia) is not present, bone marrow aspiration is not needed for most patients.2 Bone marrow aspiration may be indicated if an underlying cause is not apparent after thorough clinical evaluation and if inadequate bone marrow platelet production is suspected because large, immature platelets are absent.1,4 Thrombocytopenia is not a contraindication for bone marrow aspiration or biopsy; although bruising may occur, severe hemorrhage is uncommon.1,2,6
The proximal humerus is often selected as the aspiration site because muscle mass can be avoided and pressure can be applied to achieve hemostasis after the procedure. With immune-mediated thrombocytopenia, the bone marrow is characterized by increased total megakaryocyte numbers, as the marrow tries to respond by increasing platelet production.1 This bone marrow response should occur within three to five days of an acute thrombocytopenic episode.6 Immune-mediated megakaryocyte aplasia is rare in dogs.2
Definitive diagnosis. A clinical diagnosis of immune-mediated thrombocytopenia is usually based on finding a moderate to severe thrombocytopenia; seeing no evidence of additional hemostatic abnormalities or nonimmunologic platelet sequestration, consumption, or destruction; and finding macrothrombocytosis or microthrombocytosis.2,6,8
Demonstration of antiplatelet antibodies (Table 3) may support a diagnosis of immune-mediated thrombocytopenia but is not always necessary.8 Indirect assays (e.g. the platelet factor 3 test) detect platelet-bindable antibody in serum or plasma and have low sensitivity and specificity; these assays are no longer available.13 Direct assays (e.g. flow cytometric assays, radioimmunoassays, or ELISAs) detect platelet-surface-associated antibodies and have higher sensitivity.2,5 Assays that measure total antibody are not reliable; assays that detect platelet surface-associated immunoglobulin should be used.4 Alternatively, the antimegakaryocyte antibody test may be performed from slides of bone marrow aspirates.13 Treatment should not be withheld pending antiplatelet antibody test results. An additional limitation of antiplatelet antibody testing is that it does not differentiate primary from secondary immune-mediated thrombocytopenia.2,5,8 Secondary causes of immune-mediated thrombocytopenia must be ruled out by appropriate diagnostic testing because response to immunosuppressive therapy is the final step in confirming immune-mediated thrombocytopenia, but some patients with secondary etiologies may respond positively initially.2,6

Table 3. Laboratory Submission of Platelet-Surface-Associated Antibody Testing and Antimegakaryocyte Antibody Testing
TREATMENT
Immune-mediated thrombocytopenia is a serious and potentially life-threatening disorder.8 Mortality rates of the initial episode of thrombocytopenia or relapse range from 25% to 30%.2,8 Death is mainly from acute gi hemorrhage or euthanasia.8 Aggressive initial therapy may improve survival rates. About 25% of dogs with immune-mediated thrombocytopenia require long-term medication, and side effects are a principal reason for euthanasia in these patients.8 Recurrence after a variable period of clinical remission is common, occurring in about 40% of dogs.2,8
Initial supportive therapy
The initial management goals with immune-mediated thrombocytopenia are to stop bleeding, halt platelet destruction, and treat any concurrent disease.6 A variety of supportive measures are indicated for a patient with severe thrombocytopenia. Ideally the patient should be hospitalized and allowed to rest in a padded cage, with gentle handling to minimize trauma-related hemorrhage.2,18 Soft diets are fed to minimize gingival trauma.18,19 Avoid cystocentesis and intramuscular injections.18,19 Also avoid jugular venipuncture in patients with severe thrombocytopenia, and apply direct pressure to any venipuncture or injection site for five minutes to ensure hemostasis. Intravenous catheters are not contraindicated, but if a jugular catheter is required, placement should be done with great care, optimally after a transfusion with platelet-rich plasma.18
If the patient is hypovolemic, give crystalloids, colloids, or blood products as necessary.2 Hemodilution with synthetic colloids may decrease platelet function, so they should be used judiciously, and plasma should be considered as an alternative.18 GI protectants may be administered to patients with signs of GI hemorrhage, although the benefit is not proven.19 Drugs that decrease platelet function (e.g. nonsteroidal anti-inflammatory drugs) should be discontinued or avoided.18 Doxycycline (5 to 10 mg/kg orally or intravenously every 12 hours) may be given to treat tick-borne disease, pending the serum antibody titer results.20 If hyphema is present, administer topical 1% prednisolone acetate or 0.1% dexamethasone sodium phosphate three to four times a day with topical 1% atropine two to four times a day.18
Transfusions
In some cases, transfusions are needed to address anemia due to hemorrhage. In other instances, transfusions are given in an attempt to increase platelet numbers. Whole blood, platelet-rich plasma, or platelet concentrates are sources of platelets for transfusion. Transfusions with these products transiently boost platelet numbers, but thrombocytopenia rapidly recurs as the circulating life span of donor platelets is markedly reduced in patients with immune-mediated thrombocytopenia, often lasting only a few hours.6,8
Transfusions are also used to stop immediate bleeding in critically ill patients and may be administered before surgery (e.g. splenectomy) or other potentially invasive procedures. Fresh platelet-rich plasma is given at a dose of 10 ml/kg, which is expected to raise the platelet count by about 10,000/μl.8,18 If platelet numbers do not increase or if the increase is not sustained for at least two hours, additional transfusions are not indicated.8
Preparation of platelet-rich plasma and platelet concentrate requires special centrifugation, but these products may be purchased through commercial blood banks.18,21 Although whole blood increases the risk of volume overload and transfusion reaction, it may be given.8 Whole blood transfusion is indicated for patients with severe thrombocytopenic hemorrhage.21 Refrigeration has deleterious effects on platelet viability, so store whole blood at room temperature and use it within four hours of collection.18,21
Immunosuppressive therapy
Immunosuppressive therapy is needed to achieve the primary goal in treating immune-mediated thrombocytopenia, which is to restore normal hemostasis. Initially, it is usually adequate to achieve a platelet count > 50,000/μl, as bleeding would no longer be expected. Normalization of platelet numbers may take considerably longer.8
Glucocorticoids. Glucocorticoids are the mainstay of immunosuppressive therapy and have the most immediate effects. They suppress the mononuclear phagocytic system, decrease the affinity of antibody binding to platelets, and are the initial treatment of choice.2,6,8,19 Give prednisone or prednisolone at a dosage of 2 to 4 mg/kg/day orally. Alternatively, dexamethasone may be given at a dose of 0.2 mg/kg intravenously every 24 hours. Platelet numbers usually increase within one week of initial therapy.8
Administer the prednisone until the platelet count normalizes, and then gradually taper it. Evaluate the platelet count before any reduction in the prednisone dose, and do not taper the drug if the platelet count has decreased or has failed to increase. In some cases, normal platelet counts cannot be achieved; however, if the platelet count is adequate to prevent bleeding, tapering can be initiated. Tapering too gradually is associated with increased side effects, and tapering too rapidly may result in relapse of the immune-mediated thrombocytopenia.2
Generally, prednisone is continued at the initial immunosuppressive dose for two to four weeks. If the platelet count is adequate, the dose is reduced by 50% and continued for another two to four weeks. The dose is adjusted in this manner until the lowest effective dose that maintains an adequate platelet count is achieved. A maintenance dose of 0.5 to 1 mg/kg every 48 hours is usually well-tolerated if needed.
Side effects of prednisone, particularly at high doses, include polyuria, polydipsia, hyperventilation, and other signs of hyperadrenocorticism such as hair loss, muscle wasting, weakness, and predisposition to infection. If the platelet count decreases during the tapering of the prednisone, increase the prednisone to the last dose that effectively maintained normal platelet numbers. If the required dose of prednisone is not acceptable for long-term administration, additional immunosuppressive medications should be added.
Vincristine. Vincristine is a vinca alkaloid that transiently increases platelet numbers by stimulating transient megakaryocyte platelet release.8 It may be used to facilitate increasing the initial platelet count to a degree that the patient can be discharged earlier from the hospital.14 In addition to stimulating increased platelet release from bone marrow, vincristine may also have mild immunosuppressive effects.
Vincristine is given as a single dose of 0.02 mg/kg intravenously.8,14 Increases in platelet numbers often occur within two or three days.8 Administration of vincristine as a constant-rate infusion or incubated with a platelet transfusion has also been reported, but these routes have not been thoroughly evaluated.2,8 Vincristine is a strong vesicant if extravasated and should be given through a well-placed catheter; few other side effects are generally seen with a single dose.8,14 In vitro vincristine decreases canine platelet function, but in vivo alterations in platelet function are not well-documented.8
Other immunosuppressants. For patients with primary immune-mediated thrombocytopenia that fail to respond to glucocorticoids, additional medications may be used concurrently. Dogs with intolerable side effects from glucocorticoids may be given additional immunosuppressive medications to allow tapering or discontinuation of the corticosteroids.6,8 Azathioprine is the most common option.19 Azathioprine is an immunosuppressive agent that reduces the production of antiplatelet antibodies.8 It is given at a dose of 2 mg/kg orally every 24 hours. After two to four weeks, if the platelet count is normal, taper the azathioprine in tandem with the prednisone to the lowest effective maintenance dose.2,8 Potential side effects of azathioprine include myelosuppression, pancreatitis, hepatopathy, and predisposition to infection.8 Dogs deficient in thiopurine methyltransferase may be more susceptible to side effects.22
Alternatively, cyclophosphamide (50 mg/m2 orally on the first four days of the week for a maximum of four to five months) may be used in conjunction with prednisone for immunosuppression. Cyclophosphamide is usually less well-tolerated than azathioprine for long-term use. Potential side effects that limit its long-term use include myelosuppression, susceptibility to infection, and hemorrhagic cystitis.8 Studies in dogs with immune-mediated hemolytic anemia suggest that cyclophosphamide may no longer have a role in treating this immune-mediated blood dyscrasia.23,24
Cyclosporine (5 mg/kg orally every 12 to 24 hours) may be used instead of, or in addition to, azathioprine. The use of cyclosporine in treating immune-mediated thrombocytopenia has been described in a small number of patients, and the drug's potential side effects include vomiting, diarrhea, anorexia, and weight loss.2
Other therapeutic options
In cases refractory to standard immunosuppressive medication, other therapeutic options include splenectomy, intravenous gamma globulin administration, plasmapheresis, or treatment with danazol.
Management of immune-mediated thrombocytopenia by splenectomy has not been thoroughly investigated in a large number of dogs, and published success rates are highly variable.2,8 Blood smears and PCR testing should be done to monitor for Mycoplasma haemocanis (formerly Haemobartonella canis) and Babesia canis before and for several weeks after splenectomy.8 Immune-mediated thrombocytopenia can persist after the splenectomy through the actions of the hepatic mononuclear phagocytic system.8
Intravenous gamma globulins are given as a single infusion and are thought to competitively bind to macrophage receptors, decreasing platelet clearance by the mononuclear phagocytic system. But the effect is transient and this treatment is generally cost-prohibitive.8 In a recent report, human intravenous immunoglobulin was used at a dose of 0.28 to 0.76 g/kg intravenously.25 Other published doses for human intravenous immunoglobulin have ranged from 0.5 to 1.5 g/kg.26
Plasmapheresis is expensive and technically difficult, and its availability is limited in veterinary patients.8
Danazol (5 to 10 mg/kg orally every 12 hours) is an androgen that may decrease production of antiplatelet antibodies and decrease macrophage receptors, impairing mononuclear phagocytic system clearance of platelets.2,8 It is used concurrently with prednisone for managing immune-mediated thrombocytopenia.6,8 It may take three to six months to reach peak effect. Once the prednisone has been reduced to alternate-day dosing, the danazol dose is gradually tapered to the minimum effective dose.8 A small number of dogs with refractory immune-mediated thrombocytopenia have responded to danazol.27 Liver enzyme activities should be monitored monthly. Danazol's expense may preclude its use in large-breed dogs.8
Monitoring
Initially, monitor the platelet count every one to two weeks for the first two months. After that, recheck the count before and after any reduction in drug dose. For patients in complete remission, recheck the platelet count at three-month intervals. Monitoring the platelet count is critical because clinical signs of thrombocytopenia do not become evident until platelet numbers are severely decreased.8
VACCINATION IN AFFECTED DOGS
In patients in which immune-mediated thrombocytopenia is suspected to be secondary to vaccine administration, avoid the potentially causative vaccine or vaccines in the future. Administering distemper-hepatitis vaccines may lower platelet counts, with the peak effect one week after vaccination, so vaccination should be avoided in thrombocytopenic patients.28 For patients that have recovered from immune-mediated thrombocytopenia, we recommend administering only core vaccines and administering the vaccines at separate times. Monitoring the platelet count before and for a few weeks after vaccination is warranted.
SUMMARY
Immune-mediated thrombocytopenia is a common acquired coagulation disorder in dogs resulting from antibody-mediated clearance of platelets though the mononuclear phagocytic system. It is mainly a diagnosis of exclusion. Primary immune-mediated thrombocytopenia is confirmed by ruling out secondary disorders that can mediate platelet destruction by the immune system.
Primary immune-mediated thrombocytopenia is treated with immunosuppressive therapies, whereas secondary immune-mediated thrombocytopenia is treated by addressing the underlying disorder, often with concurrent immunosuppressive therapy. The prognosis for this disorder is guarded to good. If dogs with primary immune-mediated thrombocytopenia fail to respond, additional immunosuppressive therapy may be required, although reevaluation for an underlying cause that was not identified initially should also be considered.
Johanna Heseltine, DVM, MS, DACVIM*
Department of Small Animal Clinical Sciences
College of Veterinary Medicine
Oklahoma State University
Stillwater, OK 74078
Anthony Carr, Dr. med. vet., DACVIM (internal medicine)
Department of Small Animal Clinical Sciences
Western College of Veterinary Medicine
University of Saskatchewan
Saskatoon, SK S7N 5B4 Canada
*Dr. Heseltine's current address is Western College of Veterinary Medicine, University of Saskatchewan, Saskatoon, SK S7N 5B4, Canada.
REFERENCES
1. Mackin A. Canine immune-mediated thrombocytopenia: Part I. Compend Contin Educ Pract Vet 1995;17:353-362.
2. Lewis DC, Meyers KM. Canine idiopathic thrombocytopenic purpura. J Vet Intern Med 1996;10:207-218.
3. Scott MA. Immune-mediated thrombocytopenia. In: Kristensen AT, ed. Schalm's veterinary hematology. 5th ed. Baltimore, Md: Lippincott Williams & Wilkins, 2000;478-486.
4. Topper MJ, Welles EG. Hemostasis. In: Latimer KS, Mahaffey, EA, Prasse KW, eds. Veterinary laboratory medicine: clinical pathology. 4th ed. Ames: Iowa State University Press, 2003;99-135.
5. Boudreaux MK. Platelets and coagulation. An update. Vet Clin North Am Small Anim Pract 1996;26:1065-1087.
6. Grindem CB. Infectious and immune-mediated thrombocytopenia. In: Bonagura JD, ed. Kirk's current veterinary therapy XIII small animal practice. Philadelphia, Pa: WB Saunders Co, 2000;438-442.
7. Breitschwerdt EB. Infectious thrombocytopenia in dogs. Compend Contin Educ Pract Vet 1988;10:1177-1191.
8. Mackin A. Canine immune-mediated thrombocytopenia: Part II. Compend Contin Educ Pract Vet 1995;17:515-534.
9. Chisholm-Chait A. Mechanisms of thrombocytopenia in dogs with cancer. Compend Contin Educ Pract Vet 2000;22:1006-1018.
10. Terrazzano G, Cortese L, Piantedosi D, et al. Presence of anti-platelet IgM and IgG antibodies in dogs naturally infected by Leishmania infantum. Vet Immunol Immunopathol 2006;110:331-337.
11. Lewis DC, Bruyette DS, Kellerman DL, et al. Thrombocytopenia in dogs with anticoagulant rodenticide-induced hemorrhage: eight cases (1990-1995). J Am Anim Hosp Assoc 1997;33:417-422.
12. Cowan SM, Bartges JW, Gompf RE, et al. Giant platelet disorder in the Cavalier King Charles Spaniel. Exp Hematol 2004;32:344-350.
13. Cohn LA. Immune-mediated blood dyscrasias: clinical presentation and diagnosis, in Proceedings. Annu Am Coll Vet Intern Med Forum 2004;36-38.
14. Rozanski EA, Callan MB, Hughes D, et al. Comparison of platelet count recovery with use of vincristine and prednisone or prednisone alone for treatment for severe immune-mediated thrombocytopenia in dogs. J Am Vet Med Assoc 2002;220:477-481.
15. Bailiff NL, Norris CR. Clinical signs, clinicopathological findings, etiology, and outcome associated with hemoptysis in dogs: 36 cases (1990-1999). J Am Anim Hosp Assoc 2002;38:125-133.
16. Stockman SL, Scott MA. Hemostasis. In: Fundamentals of clinical pathology. 1st ed. Ames: Iowa State Press, 2002;157-225.
17. Brockus CW, Andreasen CB. Erythrocytes. In: Latimer KS, Mahaffey EA, Prasse KW, eds. Veterinary laboratory medicine: clinical pathology. 4th ed. Ames: Iowa State University Press, 2003;3-45.
18. Abrams-Ogg AC. Management of hemorrhage due to thrombocytopenia, in Proceedings. Annu Am Coll Vet Intern Med Forum 2002;577-579.
19. Cohn LA. Immune mediated blood dyscrasias: therapeutic options, in Proceedings. Annu Am Coll Vet Intern Med Forum 2004;39-42.
20. Plumb DC. Doxycycline. In: Plumb DC, ed. Veterinary drug handbook. 5th ed. Stockholm: Blackwell Publishing, 2005;286-289.
21. Abrams-Ogg AC. Triggers for prophylactic use of platelet transfusions and optimal platelet dosing in thrombocytopenic dogs and cats. Vet Clin North Am Small Anim Pract 2003;33:1401-1418.
22. Kidd LB, Salavaggione OE, Szumlanski CL, et al. Thiopurine methyltransferase activity in red blood cells of dogs. J Vet Intern Med 2004;18:214-218.
23. Grundy SA, Barton C. Influence of drug treatment on survival of dogs with immune-mediated hemolytic anemia: 88 cases (1989-1999). J Am Vet Med Assoc 2001;218:543-546.
24. Mason N, Duval D, Shofer FS, et al. Cyclophosphamide exerts no beneficial effect over prednisone alone in the initial treatment of acute immune-mediated hemolytic anemia in dogs: a randomized controlled clinical trial. J Vet Intern Med 2003;17:206-212.
25. Bianco D, Armstrong PJ, Washabau RJ. Treatment of severe immune-mediated thrombocytopenia with human intravenous immunoglobulin in 5 dogs, in Proceedings. Annu Am Coll Vet Intern Med Forum 2007.
26. Scott-Moncrieff JC, Reagan WJ. Human intravenous immunoglobulin therapy. Semin Vet Med Surg (Small Anim) 1997;12:178-185.
27. Miller E. The use of danazol in the therapy of immune-mediated disease of dogs. Semin Vet Med Surg (Small Anim) 1997;12:167-169.
28. Straw B. Decrease in platelet count after vaccination with distemper-hepatitis (DH) vaccine. Vet Med Small Anim Clin 1978;73:725-726.