|
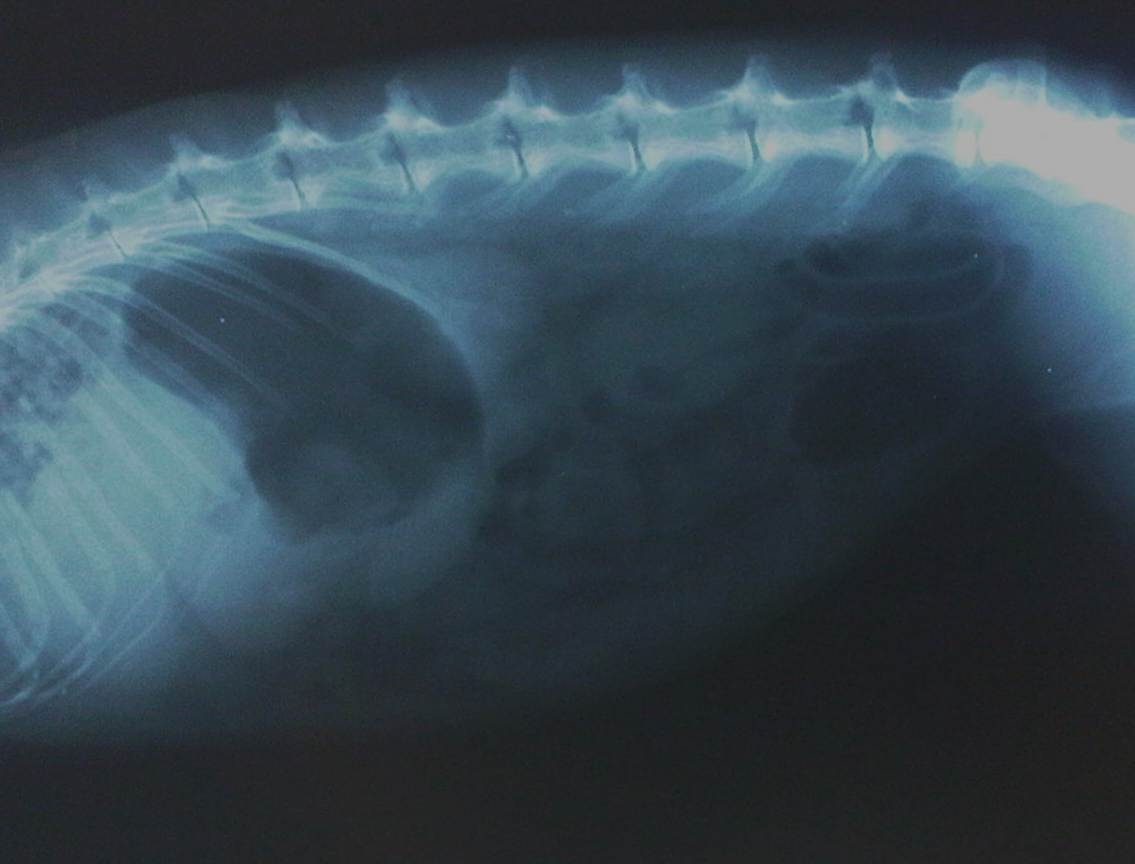
Gastrointestinal Hypomotility Disorders of the Rabbit Simon J Girling BVMS (Hons) DZooMed CBiol MSB MRCVS Aetiology of Hypomotility Disorders There are many factors which can affect gastrointestinal motility. One of the most important is the level of indigestible fibre in the diet. A lack of fibre results in reduced motility both by a lack of physical stimulation of the colon due to the absence of large fibre particles and a decreased production of volatile fatty acids in the caecum. Other causes include: increased adrenergic stimulation due to stress or pain resulting in reduced motility; and intestinal infectious diseases such as coccidiosis, E. Coli infections and entero/exotoxaemias all of which can cause varying effects on gut motility. Most cases in domestic rabbits are due to stress associated events in conjunction with a long-term dietary deficiency in indigestible fibre. The action of catecholamines on the rabbit gut can be dramatic with a single episode of pain or stress (e.g. post routine surgery, exposure to frightening noises such as fireworks or exposure to potential predators such as ferrets, dogs and cats whilst being hospitalised) resulting in significant reduction of gut motility and ultimately stomach or caecal impactions. Stomach impaction seen in cases of hypomotility usually comprises a matt of fur and food material. This has led to an assumption that fur-balls or trichobezoars in rabbits causing occlusion of the pylorus are the cause of gastrointestinal motility disorders. The stomach of the rabbit however has been shown to always contain food material and often groomed fur (Okerman, 1994). Indeed studies by Leary and others (1984) where gastric foreign bodies were artificially created using latex, rabbits affected showed no reduction in appetite or in weight. It is now widely assumed that trichobezoar formations are due to reduced gastrointestinal motility rather than the cause of it. Clinical Signs and Diagnosis A reduction in gastrointestinal motility is perhaps the most commonly seen motility disorder and can lead to impaction of food material and fur in the stomach and often the caecum. This may be palpated as an obviously distended stomach projecting beyond the caudal margin of the ribcage or a solid to doughy feeling caecum on the ventral aspect of the abdominal cavity. Onset can be rapid or more insidious. The rabbit is often dull, anorexic and lethargic, may shows signs of dehydration and is often suffering from hepatic lipidosis and ketoacidosis. Death when it occurs is usually due to liver and kidney failure. Radiographs of the abdomen may show a distended stomach with food material and an accumulation of gas in the stomach and caecum (see figure 1). This gas build-up and distension of the gastrointestinal tract can cause pain and discomfort further worsening the anorexia and hypomotility. Care should be taken to ensure there are no gut blockages as this would preclude the use of prokinetics and indicate surgery. Blood results often indicate hepatic dysfunction with elevation of hepatic associated leakage enzymes (AST is the main liver leakage enzyme in rabbits although it is also found in muscle tissue) lipaemia, metabolic acidosis and hyperglycaemia although early cases may show hypoglycaemia, and dehydration as witnessed by an elevated packed cell volume (usually over 45%). Cases where hepatic lipidosis and ketoacidosis is advanced may see damage to the kidneys and resultant elevation in urea, creatinine, phosphate and potassium. Treatment and Prevention of Hypomotility Disorders Prevention is aimed at ensuring all rabbits are fed on a high indigestible fibre containing diet which means ensuring the vast majority of the diet is comprised of either freshly grazed grass, good quality hay or dried grass. Any stressful procedure where significant catecholamine release is likely to occur e.g. post-operatively - it is beneficial to use prokinetic drugs such as metoclopramide and ranitidine as part of the therapy (see below). When treating gastrointestinal hypomotility, because there are many causes of hypomotility disorders in rabbits, care must be taken to try and accurately diagnose the initiating cause in each case (e.g. dental disease, post surgical etc). In general though, any period of anorexia in a rabbit should be treated seriously as it may be an indication of a hypomotility disorder and will ultimately lead fairly quickly to stomach and caecal impaction, hepatic lipidosis, ketoacidosis and death of the rabbit. Treatment of gastrointestinal hypomotility is geared towards encouraging normal motility, increasing appetite, reducing stress and pain and reversing any metabolic acidosis and dehydration. If some gastric impaction has occurred then oral lubricants such as liquid paraffin at 1-2ml/kg orally twice daily may aid passage of material in conjunction with fluid therapy such as oral electrolyte solutions. If moderate-severe dehydration is present then intravenous fluids should be administered. If hepatic impairment is present, then avoid using lactated Ringer’s solution as this places extra strain on the liver to metabolise lactate. Instead use 5% glucose saline and if necessary add bicarbonate to correct any recorded metabolic acidosis as for cats and dogs. Gastrointestinal pain can be countered by administering NSAIDs such as meloxicam at 0.3-0.6mg/kg and buprenorphine at 0.03mg/kg as required. Intestinal motility can be improved (assuming the clinician has confirmed that there is no blockage to the gut first) by using motility enhancers such as metoclopramide at 0.5mg/kg orally/subcutaneously twice daily in conjunction with ranitidine at 5mg/kg orally twice-three times daily. Ranitidine has the added positive effect of reducing gastric ulceration which is also common in these cases. Some evidence suggests that although an effective prokinetic, metoclopramide may only be effective in adult rabbits (Langer and Bramlett, 1997). Cisapride has been used historically as a gut motility enhancer in rabbits at 0.5mg/kg orally twice daily, but its restriction in availability in the UK has now made it very difficult to obtain. Supplemental feeding is essential to prevent or attempt to reverse hepatic lipidosis. Initially the emphasis in calorie deficient anorexic cases should be to correct the calorific deficit rapidly. This is often best achieved using a low fibre food such as a vegetable baby food (lactose free). The levels of energy required for a debilitated rabbit should approach that calculated for growing to lactating rabbits using the formula MER = k x (wt[kg])0.75 where k=200 for growth and 300 for lactation (Carpenter and Kolmstetter 2000). Therefore for debilitation, the following daily energy requirement may be used. MER = 250 x (wt[kg])0.75 Once calories have been supplied, higher fibre support formulas can then be used to provide the essential fibre for the gut flora to work on - several commercial products are available. Ad-libitum fresh good quality hay or dried grass should be offered as well as palatable foods such as dandelions, kale, and other leafy greens. To help re-populate the intestinal flora, transfaunation of caecotrophs from a healthy rabbit may aid the return of normal bowel function. The use of commercial probiotics designed for rabbits has also been advocated, and reduces the risk of transferring potential parasites and other agents to the debilitated patient. Anabolic steroids may be used in cases of hepatic lipidosis (0.5-1mg/kg once) to stimulate appetite and stop catabolism. In addition, supplements such as choline, inositol and L carnitine may all be used to support liver function and promote lipid removal from hepatocytes. Peri-Operative Use of Prokinetics As it is apparent that rabbits can suffer hypomotility of the gastrointestinal tract during periods of sympathetic nervous system stimulation, it is not unsurprising that many rabbits experience periods of hypomotility after hospitalisation or surgery/anaesthetics. For this reason it is worth considering the use of prokinetics such as ranitidine and metoclopramide pre and post operatively and during periods of hospitalisation.
This article was kindly provided by Ceva Animal Health:
References Carpenter, J.W. and Kolmstetter, C.M. (2000) Feeding small exotic animals In: Hill’s Nutrition III,(Eds Lewis, L.D., Morris, M.L. and Hand, M.S.), Mark Mervis Institute, Marceline, Missouri pp943-960 Langer, J.C. and Bramlett, G. (1997) Effects of prokinetic agents on ileal contractility in a rabbit model of gastroschisis (Abstract) Journal of Paediatric Surgery 32(4):605-608 Leary, S.L., Manning, P.J. and Anderson, L.C. (1984) Experimental and naturally occurring foreign bodies in laboratory rabbits. Laboratory Animal Science 34(1):58-61 Okerman, L. (1994) Diseases of Domestic Rabbits, 2nd Edition Blackwell Publishing, Oxford
See the complete article |
||
|
Come back soon - there'll be something shortly! |